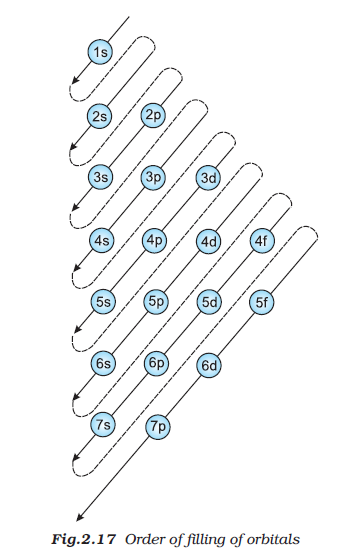
`=>` In case of scandium, 3d orbital being lower in energy than 4p is filled first.
`=>` Now in scandium, titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc 3d orbitals are progressively filled.
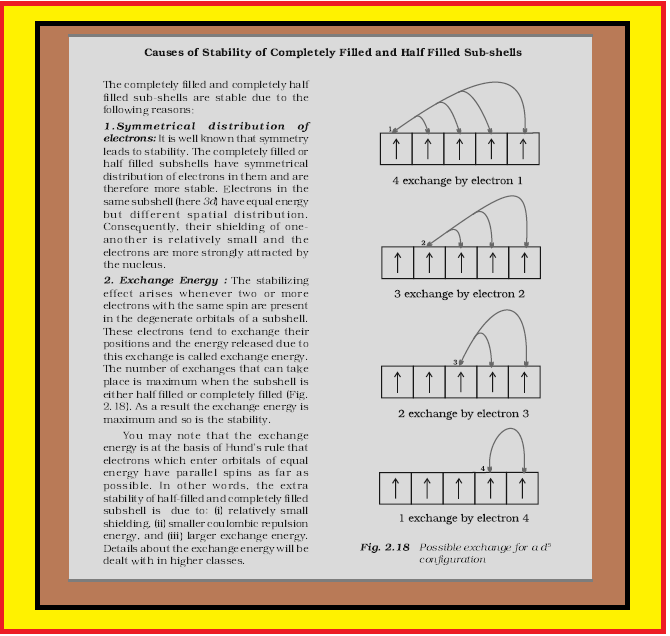
Now comes the special discussion about half filled and fully filled configuration
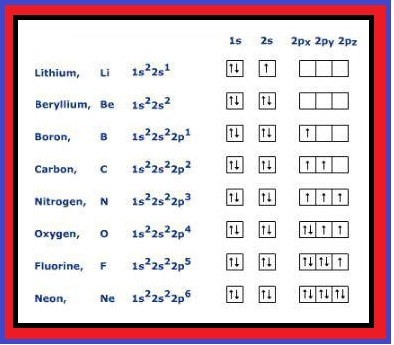
`=>` Chromium and copper have five and ten d electrons instead of four and nine electrons in their 3d orbitals. This is because `p^3`, `p^6`, `d^5`, `d^10`, `f^7`, `f^14` are either half filled or fully filled and are more stable.
`=>` With the saturation of 3d orbitals, the filling of 4p starts at gallium and is complete at krypton.
`=>` Now from rubidium to xenon the pattern of filling the 5s, 4d, 5p orbitals are similar to that of 4s, 3d, 4p orbitals.
`=>` In cesium and barium 6s conatins one and two electrons respectively.
`=.` Then from lanthanum to mercury filling of electrons takes place in 4f and 5d orbitals. After this filling of 6p, then 7s and finally 5f and 6d orbitals takes place.
`=>` The elements after uranium are all short lived and all of them are produced artificially.
`=>` In case of scandium, 3d orbital being lower in energy than 4p is filled first.
`=>` Now in scandium, titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc 3d orbitals are progressively filled.
Now comes the special discussion about half filled and fully filled configuration
`=>` Chromium and copper have five and ten d electrons instead of four and nine electrons in their 3d orbitals. This is because `p^3`, `p^6`, `d^5`, `d^10`, `f^7`, `f^14` are either half filled or fully filled and are more stable.
`=>` With the saturation of 3d orbitals, the filling of 4p starts at gallium and is complete at krypton.
`=>` Now from rubidium to xenon the pattern of filling the 5s, 4d, 5p orbitals are similar to that of 4s, 3d, 4p orbitals.
`=>` In cesium and barium 6s conatins one and two electrons respectively.
`=.` Then from lanthanum to mercury filling of electrons takes place in 4f and 5d orbitals. After this filling of 6p, then 7s and finally 5f and 6d orbitals takes place.
`=>` The elements after uranium are all short lived and all of them are produced artificially.